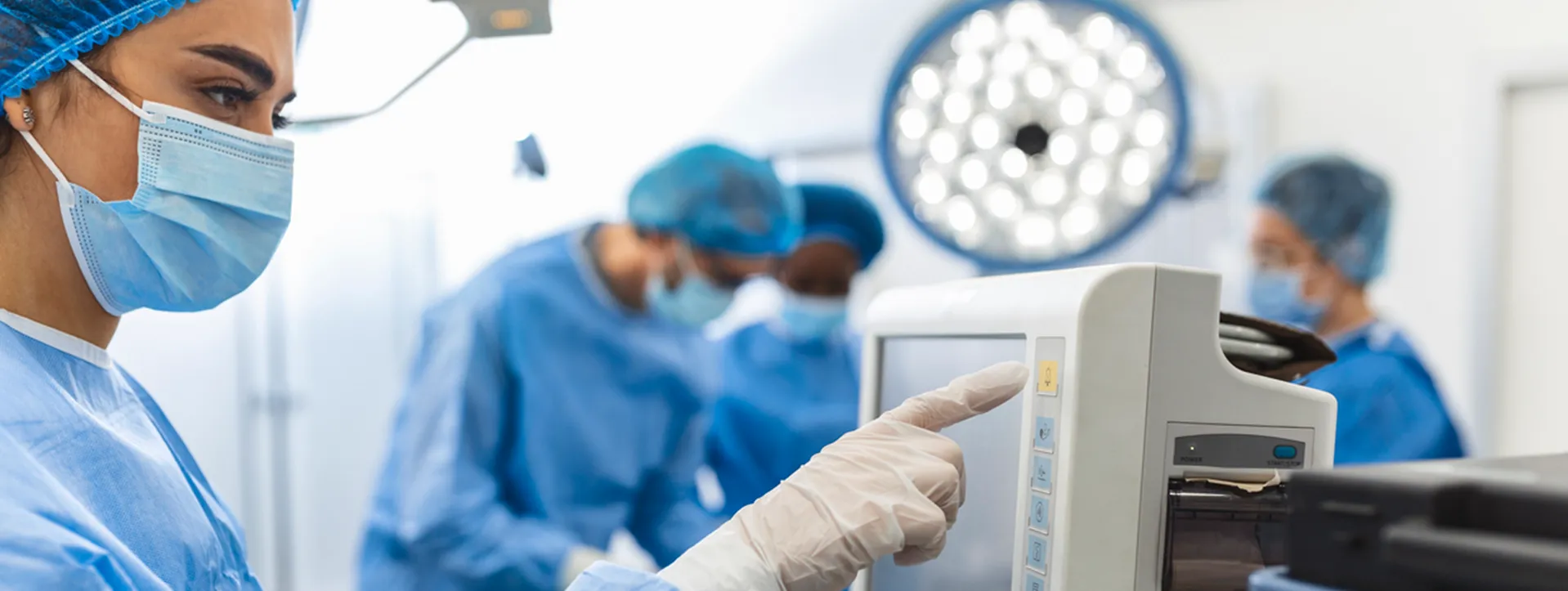
Background:
Heater-cooler devices are used during cardiothoracic surgeries, as well as other medical and surgical procedures, to warm or cool a patient to optimize medical care and improve patient outcomes. Heater-cooler devices include tanks that provide temperature-controlled fluid (usually water) to external heat exchangers or warming/cooling blankets through closed circuits.
Although the fluid in the circuits does not come into direct contact with the patient, there is the potential for contaminated fluid to enter other parts of the device or transmit bacteria through the air (aerosolize) through the device's exhaust vent, or other unsealed openings, into the environment and to the patient.
Solution:
In May 2022, the U.S. Food and Drug Administration (FDA) cleared the Spectrum Medical Quantum Heater-Cooler (Mr Frosty) and the compatible Qura Quantum PureFlow Heat Exchangers. Instead of using water, the Quantum Heater-Cooler device regulates the temperature of a glycol-based heat transfer fluid (Coolflow DTX®), which then enters the compatible PureFlow heat exchangers to ultimately regulate the temperature of a patient’s circulating blood. The Quantum Heater-Cooler's HTF demonstrated it mitigates the risk of contamination of the device by suppressing the growth of nontuberculous mycobacteria and other bacteria.
Mr Will Lansdown, Deputy Head of Perfusion – Bristol Royal Infirmary, said it was wonderful to be able to conduct extracorporeal support during cardiac surgery knowing that the risk to patient and clinicians of being infected by NTM Chimera had been eliminated. Using Mr Frosty was easy, and the noise levels were non-existent.
Key Takeaways:
FDA (USA) & CE (EU) approve Spectrum Medical Quantum Heater-Cooler & Coolflow DTX®, for use in open-heart and other major surgical operations that require blood cooling.
As a combined solution, Mr Frosty & Coolflow DTX® play a critical role in helping to protect patient welfare during surgery. The pioneering technology utilizes the benefits of a ground-breaking fluid that is not only non-toxic, but highly resistant to bacterial contamination.
A similar approach has been adopted by Chill It on behalf of Heineken and Coca-Cola. Chill It technology cools and dispenses beverages to extra-cold in seconds, drastically reducing energy consumption (60% energy savings when compared to a standard one door cooler). The Coolflow DTX® formulation used in the Chill It coolers comes into direct contact with the cans and bottles being cooled, and ultimately the hands and mouths of users. When the FDA were assessing the fluid, they had to verify that Coolflow DTX® was less toxic than the products being cooled.